2020 Volume 45 Issue 10 Pages 599-609
2020 Volume 45 Issue 10 Pages 599-609
To assess the influences of blood sampling volumes or sites on toxicological and toxicokinetic (TK) evaluations, 4-week duration animal studies and a single-dose TK study of imipramine were conducted. In the toxicological evaluation, six-week-old Sprague-Dawley rats were divided into no blood and blood sampling groups. Fifty microliters (microsampling) or 100 μL (larger sampling) of blood/time point was collected from the jugular vein (50 μL of data was reported previously as Yokoyama et al., 2020) or the tail vein 6 to 7 times on days 1/2 and in week 4. Although no parameters were affected by the 100 μL sample from the tail vein, the 100 μL jugular vein sampling decreased the red blood cell parameters in females, possibly due to hemorrhage at the sampling site. Regarding the TK assessment, 50 μL of blood/site/time point was collected at 6 time points from the tail and jugular vein of the same male rats after single oral administration of 10 or 100 mg/kg imipramine, which was selected as a representative drug with high distribution volume. Although there were no differences in the AUC0-24hr and Cmax values between the sites, the plasma concentrations at the early time points were significantly lower from the tail vein than the jugular vein. From our studies, 50 μL of jugular and tail vein microsampling did not affect the toxicity parameters or AUC/Cmax. However, appropriate toxicity considerations and/or selection of the blood sampling site may be important in the case of larger sampling volumes or blood concentration assessment.
Toxicokinetic (TK) evaluations in nonclinical safety studies are important to assess the relationship between systemic exposure and the toxicity data of drug candidate compounds, as shown by ICH S3A guidance (ICH, 1994). In general, blood sampling for TK evaluation is conducted over time at several time points from experimental animals after the administration of a candidate compound, and drug exposure analyses are conducted based on the results of blood drug concentration measurements.
Since the circulation blood volume is limited in rodents, which are generally used for toxicity studies, a satellite group for TK assessment is set in a toxicity study instead of collecting blood from the main study animals, and approximately 200 μL of blood /time point is collected for exposure analyses. Recently, it has become possible to accurately measure drug concentrations with a very small amount of blood since physicochemical analyses have improved markedly, and it is recommended to use a very small amount of over time for the blood sampling method (so-called microsampling) from the main study animals for TK assessment as described in ICH S3A Q&A (ICH, 2017). The Q&A (ICH, 2017) defines microsampling as a method to collect a very small amount of blood (typically ≤ 50 μL). Microsampling contributes to the benefits of the 3Rs of animal experiments by reducing distress in animals by minimizing the total volume of blood collected from individual animals and also by reducing or eliminating the need for TK satellite animals. Moreover, a scientific advantage of microsampling is that the relationship between the safety data and drug exposure can be directly evaluated in the same animal. However, data to confirm the influences of blood sampling from the main study animals on toxicological evaluations or TK assessments are limited so far and are considered to prevent the progression of incorporating microsampling into toxicity studies.
We previously investigated the effects of microsampling in a toxicological evaluation in a 4-week repeat-dose toxicity study in 6-week-old Sprague-Dawley (SD) male and female rats at the start of administration in four testing facilities. Blood samples (50 μL/time point) were collected from the jugular vein without anesthesia for up to 7 time points (6 time points on days 1/2 and 7 time points on days 27/28). As a result of the study, there were no consistent toxicological parameter changes between the facilities, and the effects of microsampling with 50 μL/time point for up to 7 time points on the toxicological evaluation were extremely small (Yokoyama et al., 2020). Similar results were noticed in other studies. Powles-Glover et al. (2014) reported that microsampling (6 × 32 μL) from the tail vein was performed in 10-week-old male and female rats on days 1 and 14, and then clinical pathology and histopathology were examined at termination on day 15. As a result of the study, there were no changes in the examined parameters except for a decrease in hemoglobin concentration in females and an increase in monocytes in males. Caron et al. (2015) reported that microsampling (6 × 50 μL) from the saphenous vein or tail vein was performed in 8-week-old female rats on days 1 and 28, and examinations were performed on days 2 and 29 (the day following blood sampling). As a result of the study, there were no effects from blood sampling on the toxicological parameters except for a few (fibrinogen and AST) on day 2. Therefore, it can be concluded that microsampling (≤ 50 μL/time point) is generally robust enough to be incorporated into toxicity studies.
However, in our previous study, subcutaneous hemorrhagic lesions in the sampling site of the neck due to blood collection were observed in some rats, and it was suggested that adding blood loss from the local blood collection site to the actual blood sample volume might exacerbate the changes in toxicological parameters (Yokoyama et al., 2020). According to the abovementioned reports of Powles-Glover et al. and Caron et al., the effects of blood sampling on hematological parameters, including hemoglobin, hematocrit and red blood cell (RBC) counts, were certainly noted in blood sampling of the 6 × 200 μL group. A blood sampling volume of more than 50 μL may be required due to marked blood loss, low sensitivity of the analytical method for TK analysis, or securing blood samples for remeasurement, such as incurred sample reanalysis. Some unclear points remain regarding the effects of blood sampling volume on toxicological evaluation. In general, it has been reported that if a recovery period is applied for 1, 2 or 3 weeks, 7.5%, 10-15%, and 20% of the circulating blood volume in rats can be collected serially within 24 hr. Moreover, the mean circulating blood volume in rats has been described as 64 mL/kg (Diehl et al., 2001). Therefore, the assumed body weight of a 6-week-old rat, which is usually used at the start of administration in a general toxicity study, is 200 g for males and 150 g for females, and the circulating blood volume has been calculated to be 12.8 and 9.6 mL, respectively. The theoretical blood volume that can be collected has been calculated to be 0.96 mL for males and 0.72 mL for females, assuming 7.5% of the circulating blood volume. Therefore, the acceptable sampling volume is considered to be approximately 100 μL/time point when 6 to 7 serial blood samplings occur in TK analysis using 6-week-old rats. However, the effects of blood sampling without a recovery period on the toxicological parameters are unknown, and a maximum sampling blood volume that can be incorporated into toxicity studies is unclear.
Regarding technical feasibility, the main sites of serial blood sampling in rats include the jugular vein, tail vein, and saphenous vein, as well as the sublingual vein and the orbital venous plexus (Diehl et al., 2001). Among them, blood sampling from the jugular vein and the tail vein are considered less invasive to animals and can be easily collected without anesthesia, but there are no detailed reports comparing the effects of these sampling sites on toxicological evaluations. There are some studies on the effects of the differences in the blood sampling site on pharmacokinetic (PK) analysis. Vangala et al. (2015) reported that there were no clear differences when comparing the PK parameters of dapsone (diaphenylsulfone) among the samples collected from the orbital vein, jugular vein and saphenous vein. Korfmacher et al. (2015) reported that differences in the changes in blood drug concentrations of some compounds were observed in the PK study when the PK parameters of 5 compounds were compared between jugular vein cannula sampling and capillary microsampling from the tail vein. The differences in sampling site, i.e., sampling from the jugular vein, which is considered to have large blood flow, and the tail vein, which is located at the periphery, may affect the TK assessment and, in particular, compounds that may have a high volume of distribution. We considered it necessary to verify the effects of the sampling sites on TK parameters for a high partition coefficient/distribution volume.
In this investigation, we first examined the effects of blood sampling volume and sampling site (the jugular vein and tail vein) on toxicity evaluation in a regular 4-week assessment, and then in a TK assessment at the toxic drug doses by single administration. The effects of over-time blood sampling with a sampling volume of 100 µL/time point from the jugular vein and 50 or 100 µL/time point from the tail vein, which were not reported in our previous report (Yokoyama et al., 2020), on the toxicological parameters were investigated as the toxicological evaluation. In the TK assessment, a tricyclic antidepressant, imipramine, was selected as the test substance because its volume of distribution (11.0-18.2 L/kg) is high, suggesting potential high tissue localization, which may cause differences in drug concentrations between systemic and peripheral blood (Nagy and Johansson, 1975; Gram et al., 1976; Sutfin et al., 1988). After a single oral dose of imipramine at approximately toxic doses of 10 or 100 mg/kg (Uehara et al., 2010), microsampling was performed from the jugular vein or the tail vein in the same animal with a sampling volume of 50 µL/time point. We examined the effects of the blood sampling sites on the plasma drug concentrations as well as their AUC and Cmax values.
We conducted three experiments, two 4-week duration toxicity studies (Exp 1 for jugular vein sampling and Exp 2 for tail vein sampling) and one TK study (Exp 3), in order to investigate the influences of (micro)sampling on the toxicological and TK parameters in rats. These experiments were conducted at the Research Institute for Bioscience Products and Fine Chemicals, Ajinomoto Co., Inc. from 2017 to 2019 with reference to the test guidelines for toxicity studies (ICH S4) and TKs (ICH S3A). The procedures for animal experiences were approved by the Ethics Committee of the Innovation Research Institute based on the guidance for animal care and use of the testing facility. Only imipramine measurements of plasma samples were performed in the National Institute of Health Sciences.
Test substanceImipramine hydrochloride (lot no. WDN5600, Wako Pure Chemical Industries, Ltd., Osaka, Japan) was suspended in 0.5% methylcellulose solution (lot no. TWN7021, Wako Pure Chemical Industries, Ltd.) to achieve concentrations of 2 and 20 mg/mL as dosing solutions for Exp 3.
Animal and husbandryFive-week-old Crl:CD(SD) rats were purchased from Charles River Laboratories Japan, Inc. (Kanagawa, Japan). The animals were quarantined and acclimated for one week and divided by stratified randomization into three body weight matched groups with 6 male and 6 female rats in the Exp 1 and Exp 2 groups and divided into two groups of 3 male rats for the Exp 3 group. The animals were housed individually in bracket-type metallic cages in the animal room, which was maintained at a temperature between 20-26°C and at a relative humidity between 30-70% with a flow of 10-15 air changes per hour. Lighting was controlled to give a 12-hr light cycle from 7 a.m. to 7 p.m. All rats had free access to tap water through a water bottle and a commercial pellet diet (CRF-1, Oriental Yeast Co., Ltd., Tokyo, Japan) in a metallic basket.
Observation and examinations Clinical observation, body weight, food and water consumptionThe clinical signs of the animals were observed daily. Body weight and food and water consumption were measured once a week or more in Exp groups 1 and 2.
OphthalmologyOphthalmologic examinations were conducted during the pretesting period and in week 4 in the Exp 1 and 2 groups. First, the eyes and peribulbar structures were observed macroscopically. After instillation of a mydriatic (Mydrin-P, Santen Pharmaceutical Co., Ltd., Osaka, Japan), the anterior portion of the eyes, optic media and ocular fundus were examined using a binocular indirect ophthalmoscope (Omega 200, Heine Optotechnik GmbH & Co. KG, Germany) and/or a slit-lamp (KOWA SL-15, Kowa Company, Ltd., Aichi, Japan).
Clinical pathologyAnimals were anesthetized with isoflurane, and blood samples of approximately 3.5 mL for hematology and blood chemistry examination were collected from the postcava at terminal necropsy after overnight fasting (approximately 18 hr. Approximately 1 mL of the blood was dispensed into blood collection tubes containing 3.8% sodium citrate and then centrifuged to obtain a plasma sample that was used for the hematological test. Approximately 1-1.5 mL of the blood was dispensed into blood collection tubes containing EDTA-2K and used for the coagulation test. These blood samples were analyzed using a hematology analyzer (ADVIA2120, Siemens Healthcare Diagnostics K.K., Tokyo, Japan) and an automated blood coagulation analyzer (CS2000i, Sysmex Corporation, Hyogo, Japan) for the following hematological parameters: RBCs, MCV, PLTs, HGB, HCT, MCH, MCHC, WBCs, differential WBCs (count and ratio), RETICs (count and ratio), PT, APTT and Fbg. Approximately 1 mL of the 3.5 mL blood sample was divided into a heparinized blood collection tube and was then centrifuged to obtain a plasma sample. Plasma samples were analyzed using an automated clinical chemistry analyzer (TBA-120FR, Toshiba Co., Tokyo, Japan) for LDH, CPK, AST, ALT, ALP, T-BIL, CRE, BUN, TGs, CHO, PL, GLU, Na, K, Cl, Ca, IP and TP and using an automated electrophoresis analyzer (Epalyzer Junior, Helena Laboratories, Saitama, Japan) for the plasma protein fraction. Urinary samples were obtained by 24-hr urine collection using a metabolic cage during week 4. A portion of the urine sample was obtained at 4 or 5 hr after the start of overnight collection as a fresh urine sample. The fresh urine samples were analyzed for pH, glucose, protein, occult B, ketones, BIL and urobilin using a urine chemistry analyzer (CLINITEK Advantus, Siemens Healthcare Diagnostics Inc., Tokyo, Japan) or urine reagent strips (Multistix, Siemens Healthcare Diagnostics Inc., Tokyo, Japan). Cumulated urine samples were analyzed for specific gravity using a urine specific gravity refractometer (UG-D, ATAGO Co., Ltd., Tokyo, Japan) and for Na, K and Cl using an automated electrolyte analyzer (EA07, A&T Corporation, Kanagawa, Japan). Total urine volume was calculated as the sum of manually measured fresh and cumulated urine volumes.
Pathological examinationsOn the last day of the 4-week observation period, i.e., day 29 of Exp 1 and day 31 of Exp 2 (on the day following the last (micro)sampling), animals were anesthetized with isoflurane and euthanized by exsanguination from the postcava after blood sampling for clinical pathology. Macroscopic examination was conducted, and then the major organs, i.e., the brain, pituitary, submandibular gland, thymus, heart, liver, spleen, kidneys, adrenal glands, testes and ovaries, were weighed, and the relative organ weights were calculated based on the body weight at necropsy in both experiments. In Exp 1, the lungs, heart, liver, spleen, kidneys, adrenal glands, sternum including bone marrow, and other gross abnormal lesions were examined histopathologically.
Blood sampling for assumed TKsBlood samples for assumed TKs were collected from the jugular vein in Exp 1 using a heparinized 1 mL syringe and a 27G injection needle on days 1-2 and 27-28. In Exp 2, blood sampling was conducted from the tail vein using a blood collection capillary and a 25G winged needle on days 1-2 and 29-30. In both experiments, 9:30 a.m. was regarded as the administration time (no drug administration in this study), and blood sampling was conducted at 0.5, 1, 2, 4, 8 and 24 hr after administration on day 1 and at 0 (predose), 0.5, 1, 2, 4, 8 and 24 hr after administration in week 4 for all animals without anesthesia. The sampling volume was 100 μL/time point from the jugular vein and 50 or 100 μL/time point from the tail vein. Data on the 50 μL/time point from the jugular vein were already published in our previous study (Yokoyama et al., 2020).
TKIn Exp 3, three male rats per group were orally administered imipramine at a single dose of 10 or 100 mg/kg. Blood samples were collected from two sites in the same animal, i.e., first sampling from the tail vein and then sampling from the jugular vein, at 0.5, 1, 2, 4, 8 and 24 hr after administration without anesthesia. For blood sampling from the tail vein, a blood collection capillary and a 25G winged needle were used. For blood sampling from the jugular vein, a heparinized syringe with a 29G needle (BD low-dose 3/10 mL 29G, Nippon Becton Dickinson Company, Ltd., Tokyo, Japan) was used. The volume of blood sampling was 50 μL/site/time point (a total of 100 μL/animal/time point). The plasma concentrations of imipramine were determined by a reversed-phase liquid chromatography-mass spectrometry (LC-MS) system (Ultimate 3000 RSLC and TSQ Quantiva, Thermo Fisher Scientific, Waltham, MA, US). The range of calibration standards was set from 1 ng/mL to 1000 ng/mL with 7 points. A 10 µL plasma sample was mixed with 490 μL of H2O/acetonitrile (1/4, v/v) with 0.1% formic acid, containing imipramine-d4 (Toronto Research Chemicals, North York, ON, Canada) as an internal standard. Subsequently, mixed samples were filtered with FastRemover Phospholipid (GL Science, Tokyo, Japan) to remove proteins and phospholipids. The resulting solution (5 μL) was subjected to LC-MS analysis. An InertSustainSwift C18 column (1.9 μm, 2.1 × 50 mm; GL Science) was used to separate imipramine, and the column oven temperature was set to 50°C. The solvent compositions of the mobile phase were water with 0.1% formic acid for solvent A and methanol with 0.1% formic acid for solvent B, and the mobile phase was pumped through at a flow rate of 400 μL/min with a multistep gradient. The MS was operated in heated ESI positive ion mode, and the detection of imipramine and imipramine-d4 was conducted using selected reaction monitoring at the transition of 281.172 > 86.11 for imipramine and 285.212 > 86.11 for imipramine-d4. The peak areas of imipramine and imipramine-d4 were quantified with TraceFinder software (version 3.3, Thermo Fisher Scientific) and subjected to the calculation of plasma imipramine. The Cmax, Ct, Tmax and AUC0-24hr were calculated as the TK parameters.
Statistics and data analysesIn Exps 1 and 2, the mean and standard deviations of the quantitative data on body weight, food consumption, water consumption, organ weight (absolute and relative weight), and hematology, blood chemistry and urinalysis were calculated and analyzed by an algorithm as follows: Bartlett’s test was performed for equality of variance (two-sided test, judged significant if p < 0.01). When the variance was homogeneous, significant differences in the means between the control group and blood sampling groups were analyzed by Dunnett’s multiple comparison test (two-sided test, judged significant if p < 0.05). If the variances were not homogeneous, the average of the ranks between the control group and blood sampling groups was analyzed by Steel’s multiple comparison test. In Exp 1, the data of the control, 50 and 100 μL/time point groups were analyzed by the multiple comparison test, though the data from the control and 50 μL/time point groups were previously published (Yokoyama et al., 2020).
In Exp 3, the mean and standard deviations of plasma drug concentration, Cmax, Tmax, and AUC0-24hr were calculated. An F-test was conducted for equality of variance. The differences in the means between blood samples from the jugular vein and tail vein at the same dose level were analyzed by Student’s t-test when the variance was homogeneous and by Aspin-Welch’s t-test when the variances were not homogeneous.
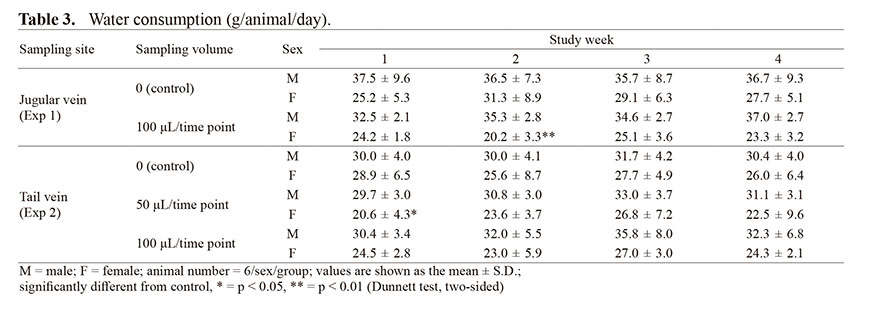
All animals survived to the end of the observation period, and no abnormal general conditions were observed in any experiments in the Exp 1 and 2 groups (Tables 1 to 3). Although significant decreases in food consumption and water consumption in week 2 of the jugular vein sampling group were detected in females of the 100 μL/time point groups, these observations were not observed at the other weeks and were derived due to high values in the control groups. Therefore, this change was judged as accidentally transient and less significant. Similarly, at the 100 μL/time point groups, there were no influences on body weight, food consumption, or water consumption in the tail vein sampling groups. Since 50 μL of sampling/time point from the jugular vein did not affect these parameters in our previous study (Organization B, Yokoyama et al., 2020), no effects in the 100 μL sampling/time point were suggested for these 3 items, regardless of the two sampling sites.


 Ophthalmology
Ophthalmology
No influences were observed in the ophthalmology examinations in either the 50 μL or 100 μL jugular vein or tail vein samplings in the Exp 1 and 2 groups shown in this and our previous studies (Yokoyama et al., 2020).
Hematology and clinical chemistryIn the hematological examination (Table 4), RBCs, HGB and HCT were significantly decreased in females, and fibrinogen was increased in males in the Exp 1 group. The monocyte ratio decreased, and the eosinophil ratio and eosinophil counts increased in females in the 100 μL/time point tail vein sampling group (Exp 2) (data not shown). In the blood chemistry examination, TGs decreased in females in the Exp 1 group, GLU and α1-globulin increased, α2-globulin decreased in males at 100 μL/time point, and TGs, CHO, PL and Ca increased in females in the 100 μL/time point tail vein sampling group (Exp 2) (Table 5, partial data not shown). On the other hand, 50 μL sampling/time point from the tail vein did not affect the hematology and clinical chemistry parameters, as in our previous study on jugular vein microsampling (except for serum creatinine in females; Yokoyama et al., 2020). There were no differences in the urinary parameters in either sampling volume in the Exp 1 and 2 groups (data not shown). However, since all changes observed in the hematology and clinical chemistry examinations of the Exp 1 and 2 groups except for the changes in RBC parameters of the Exp 1 group were within the range of the historical control data of the testing facility, these changes were considered to be within the physiological range and have no toxicological significance.

 Pathological examinations
Pathological examinations
At necropsy, hemorrhagic lesions were observed in the subcutaneously in the neck of all rats, and in the thoracic cavity of one female in the Exp 1 group, and this was considered to be related to the blood sampling site. Absolute and relative thymus weights decreased with statistically significance in females in the Exp 1 group (Table 6). Since these organ weight changes were within the range of the historical control data of the testing facility and no relevant histopathological changes were observed in the Exp 1 group, these changes were considered to be within the physiological range and have no toxicological significance. No histopathological changes in the examined tissues/organs suggested that no influence was observed by 100 μL jugular vein sampling in the Exp 1 group.

There were no abnormal findings in the necropsy and organ weights in after tail vein sampling for either 50 or 100 μL/time point, suggesting no influence of the sampling volume/site in the Exp 2 group. Therefore, histopathological examination was not conducted in the Exp 2 group. Additionally, 50 μL/time point from the jugular vein showed no influence on organ weights (Organization B, Yokoyama et al., 2020).
Blood sampling for assumed TKsThere were no deviations from any scheduled time points of blood sampling in the Exp 1 group. Deviations from the scheduled blood sampling time (within 1 to 5 min from each sampling point) occurred at six time points in males and two time points in females on day 1 and four times in females on day 29 in the Exp 2 group. However, since the expected blood volume was collected at each time point, evaluation of the blood sampling effect on the toxicity was considered to be possible. The frequency of deviation from the time point in Exp 2 was calculated as 3.8% (12/312).
TKIn the TK study, blood samplings were successfully performed at all scheduled time points except for 11 time points in total, and there were no missing data. Since each deviation from the scheduled sampling time was small and the difference in blood collection time between the sampling sites (i.e., jugular vein and tail vein) in the same animal was almost within 2 min, these deviations were considered to have no effect on the TK analysis. The frequencies of deviation from the scheduled time point were calculated as 13.9% (5/36) for tail vein sampling and 16.7% (6/36) for jugular vein sampling. However, since the 5 of 6 deviations from the jugular vein sampling were caused by a deviation from the scheduled time point of sampling from the tail vein, the actual deviation frequency for the jugular vein was calculated as 2.8% (1/36).
The Cmax and AUC0-24hr increased in a dose-dependent manner in both blood sampling site groups. The plasma concentrations of imipramine for up to one hour postdose at a dose of 10 mg/kg and at up to 2 hr postdose at the dose of 100 mg/kg from the tail vein blood sampling groups were significantly lower than those from the jugular vein blood sampling groups. However, there were no significant differences in the AUC0-24hr, Cmax and Tmax values between the jugular vein sampling and tail vein sampling groups (Table 7).

In the toxicological evaluation, no parameters were affected after sampling of up to 100 μL from the tail vein, but the 100 μL jugular vein sampling decreased the red blood cell parameters in females. On the other hand, there were no differences in the AUC0-24hr and Cmax values between the sites, but plasma concentrations at early time points were significantly lower from the tail vein than the jugular vein in the TK assessment. Thus, sampling from the jugular vein is considered more limited for toxicological evaluation use than sampling from the tail vein in view of the allowed sampling volume. Furthermore, blood concentration data from the jugular vein and tail vein are suggested not to be mixed for TK evaluation for compounds with a high volume of distribution.
Since hemorrhagic lesions were observed subcutaneously in the neck of all rats in the jugular vein blood collection group and no degenerative changes in the hematopoietic organs were observed in the histopathological examination, the cause of the RBC parameter changes was considered to be that further blood loss occurred at the local sampling site in addition to the volume of actual blood collected from the jugular vein. Note that degrees of hemorrhage at the sampling sites (jugular vein) were similar between the results of this study and of Organization B in Yokoyama et al., 2020. Because hemorrhagic lesions at the site of blood sampling is supposed to be observed more or less depending on the skill level of the facility, the effect of blood loss may additionally appear or depress.
The gender differences in these hematological changes were considered to have a high impact on blood loss for female rats, since females have a lower total blood volume than males. Indeed, the ratio of total sampling blood volume to the circulation blood volume calculated based on the body weights in week 4 was calculated as 2.8-2.9% for males and 4.5-4.7% for females in the 100 μL sampling/time point groups (i.e., the ratio of females was higher than that of males (Table 8)). The blood sampling volume that can be serially collected within 24 hr in rats without toxicological significance is described as 7.5% of the circulation blood volume if a 1-week recovery period is set (Diehl et al., 2001). Since the sampling blood volume in this study was lower than the maximum tolerable sampling blood volume (although only 1 day recovery was applied between blood sampling and necropsy in this study) and no clear influences were observed at up to 100 μL/time point from tail vein sampling, 100 μL sampling/time point was suggested to be tolerable for this typical rat study. However, since blood loss from the sampling site was added to the actual blood volume taken from the jugular vein, decreases in the RBC parameters due to blood loss were considered to be particularly noted in females in the 100 μL/time point jugular vein sampling group. No changes in RBC parameters were observed at the 50 μL/time point (Organization B is the current organization data), and the ratio of sampling blood volume was 2.2-2.5% of females in the microsampling jugular vein group (Yokoyama et al., 2020). Therefore, the ratio of the maximum blood sampling volume to the circulating blood volume in toxicity studies for jugular vein sampling was considered to be desirable up to 3%. In the case of sampling from the tail vein, if the ratio of theoretical blood sampling volume to the circulation blood volume was below 5%, there were no effects on the toxicological parameters in this study. Practically and with a good sampling technique, sampling up to 100 μL/time point could be incorporated into general 4-week toxicity studies when starting with 6-week SD rats.

According to our current results, 100 µL/time point blood sampling from the jugular vein from females in toxicity studies would be considered undesirable. To solve this issue, improvement of sampling techniques to reduce bleeding, e.g., the use of thinner needles, prolonged compression time of the blood sampling site, or modification of the method for the sampling from the cervical blood vessels through the chest muscles in order to align the volume of blood loss from the animal with the actual sampling volume could avoid the observed toxicological influences.
Regarding the effects of sampling site differences on the TK analysis, the plasma concentrations of imipramine in the tail vein blood samples at up to 1 (10 mg/kg) or 2 (100 mg/kg) hr postdose were significantly lower than those of the jugular vein blood samples. Since the distribution of imipramine to the tail vein could be slower than that to the jugular vein, some differences in the blood drug concentrations were considered to occur at an early point after administration. However, since there were no significant differences in any of the TK parameters (Cmax, Tmax and AUC0-24hr) important for assessing drug exposure between the two blood sampling sites (jugular vein and tail vein), it was concluded that there is no significant influence between sampling sites for the TK evaluation when one sampling site is used throughout the assessment period. Korfmacher et al. (2015) compared the PK parameters of fluoxetine, which has a large volume of distribution (distribution volume of 20-42 L/kg) (Johnson et al., 2007), which is similar to imipramine, between jugular vein cannula sampling and capillary microsampling from the tail vein. As a result of the study, lower blood concentrations from the tail vein than from the jugular vein were observed. In contrast, glipizide, which is a low volume of distribution compound with a Vd of 0.13 L/kg, showed no differences in the blood concentrations due to sampling sites (Ostman et al., 1981). Therefore, the extent of drug tissue distribution was considered to be related to the differences in blood concentrations between sampling sites, especially early after oral administration.
In order to clarify the differences in the technical features of each blood sampling site in addition to the evaluation of the effects on toxicology and TK, we performed blood sampling without restriction on the number of punctures in each sampling, even when the sampling time exceeded the acceptable range of scheduled time points in the experiments. We compared the frequency of deviation from the scheduled blood sampling time between both sampling sites in order to assess the appropriateness of each sampling site. As a result, several deviations occurred in tail vein sampling in both the toxicity study and TK analysis, and the frequency of deviation from the total time point was 3.8% in Exp 2 and 13.9% in Exp 3. However, the actual deviation frequency of the jugular vein sampling in Exp 3 was 2.8%. Although the reason for the high frequency of deviation in the tail vein sampling is not clear, damage to the blood vessels by repeated puncture may decrease the blood flow, leading to the success rate of blood sampling being considered reduced. Blood sampling from the jugular vein, by contrast, was considered to be a reasonable method for serial blood sampling, although blood leakage from the sampling site should be considered.
In summary, although blood sampling from the jugular vein was previously limited to use in toxicity studies due to its high invasiveness to the whole body, it is considered to be an appropriate method in TK analysis with up to 7 time points with 50 μL/time point since it had few missing samples and the blood leakage from the sampling site did not significantly affect the toxicological results. Blood sampling from the tail vein may have caused missing samples, but due to the low invasiveness to the whole body, it can be incorporated at up to 7 time points at 100 μL/time point into a toxicity study, and it was considered to be a suitable method for toxicological evaluation; in particular, when a larger blood sampling volume is needed. In the case of drugs with a high partition coefficient/distribution volume, some differences were suggested in the blood concentrations early after oral administration between samples from the tail vein and the jugular vein, but it was found that there were no differences in the major parameters between the two analyzed sites. Our present findings make it possible to select an appropriate blood sampling method based on the necessary blood volume and the compound profile and hopefully will contribute to the further utilization of microsampling methods in toxicological studies.
This work was supported in part by AMED under grant numbers JP17ak0101073j0001 and JP18ak0101073j0002.
Conflict of interestNorimichi Hattori and Asuka Takumi are employees of Ajinomoto Co., Inc. No other conflicts of interest are declared for this work.